Pharmaceutical Supply Chain Management Through ERP: Complete Guide, Features and Details
The pharmaceutical industry operates within a complex and highly regulated environment. From research and development to manufacturing, distribution, and sales, the supply chain is intricate and demanding. Managing this chain effectively is crucial for ensuring patient safety, regulatory compliance, and profitability. In today’s competitive market, pharmaceutical companies are increasingly turning to Enterprise Resource Planning (ERP) systems to streamline their operations and gain a competitive edge.
ERP systems offer a comprehensive solution for integrating various business processes, including inventory management, production planning, quality control, regulatory compliance, and financial accounting. By centralizing data and automating workflows, ERP systems provide real-time visibility into the entire supply chain, enabling pharmaceutical companies to make informed decisions and respond quickly to changing market demands. This is particularly important in an industry where delays and errors can have serious consequences for patients and the business itself.
This article aims to provide a complete guide to pharmaceutical supply chain management through ERP systems. We will explore the key features and benefits of ERP in this context, discuss the challenges of implementation, and provide practical insights for choosing the right ERP solution for your organization. Having been involved in several ERP implementations within pharmaceutical companies, I can attest to the transformative power of these systems when implemented and managed correctly. Let’s dive in!
Understanding the Pharmaceutical Supply Chain
The pharmaceutical supply chain is unique due to its inherent complexities and strict regulatory requirements. Unlike other industries, pharmaceutical companies must adhere to stringent guidelines set by regulatory bodies like the FDA (in the US) and EMA (in Europe) at every stage of the supply chain. This includes ensuring product quality, safety, and efficacy, as well as maintaining traceability and preventing counterfeiting.
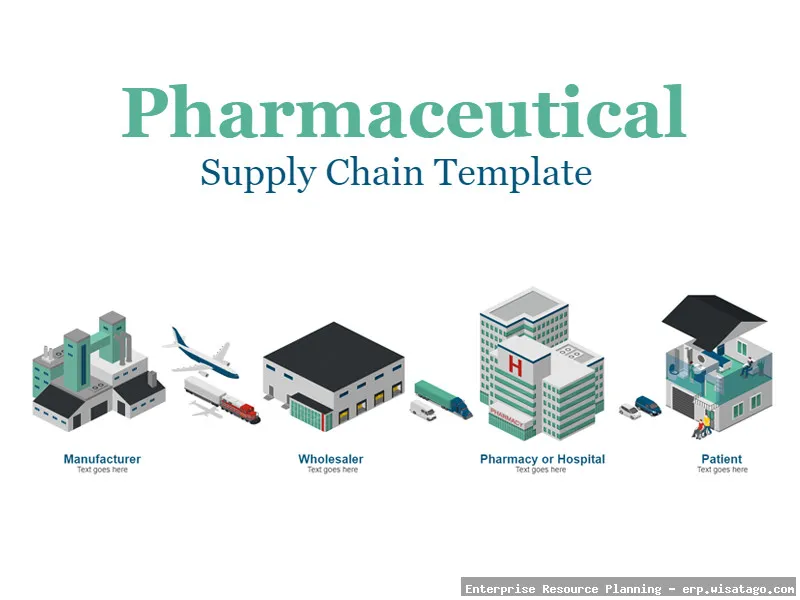
Key Stages of the Pharmaceutical Supply Chain
The pharmaceutical supply chain typically involves the following key stages:
- Research and Development (R&D): Discovering and developing new drugs, including clinical trials and regulatory submissions.
- Raw Material Sourcing: Procuring active pharmaceutical ingredients (APIs), excipients, and packaging materials from reliable suppliers.
- Manufacturing: Producing drugs in accordance with Good Manufacturing Practices (GMP) to ensure quality and consistency.
- Packaging and Labeling: Packaging drugs appropriately and labeling them with accurate information, including dosage, expiration date, and storage conditions.
- Distribution: Transporting drugs from manufacturing facilities to wholesalers, distributors, pharmacies, and hospitals.
- Sales and Marketing: Promoting and selling drugs to healthcare professionals and patients.
- Pharmacovigilance: Monitoring the safety and efficacy of drugs after they are released to the market.
Challenges in Pharmaceutical Supply Chain Management
Managing the pharmaceutical supply chain presents several challenges, including:

- Regulatory Compliance: Meeting stringent regulatory requirements, such as GMP, GDP (Good Distribution Practice), and track-and-trace regulations.
- Counterfeit Drugs: Preventing the entry of counterfeit drugs into the supply chain.
- Temperature Sensitivity: Maintaining the cold chain for temperature-sensitive drugs.
- Demand Forecasting: Accurately forecasting demand to avoid shortages or overstocking.
- Inventory Management: Optimizing inventory levels to minimize waste and reduce costs.
- Supply Chain Disruptions: Managing disruptions caused by natural disasters, geopolitical events, or supplier issues.
ERP Systems for Pharmaceutical Supply Chain Management: An Overview
ERP systems provide a centralized platform for managing all aspects of the pharmaceutical supply chain, from raw material sourcing to distribution and sales. By integrating various business processes and providing real-time visibility into the supply chain, ERP systems help pharmaceutical companies address the challenges mentioned above and improve their overall efficiency and profitability.
Key Features of ERP Systems for Pharmaceuticals
Here are some of the key features offered by ERP systems tailored for the pharmaceutical industry:
- Inventory Management: Real-time tracking of inventory levels, batch management, and expiry date tracking.
- Production Planning: Demand forecasting, material requirements planning (MRP), and capacity planning.
- Quality Control: Quality inspection, batch testing, and non-conformance management.
- Regulatory Compliance: Support for GMP, GDP, and track-and-trace regulations.
- Financial Accounting: General ledger, accounts payable, accounts receivable, and cost accounting.
- Supply Chain Collaboration: Integration with suppliers, distributors, and other partners.
- Sales and Marketing: Customer relationship management (CRM), order management, and pricing.
- Warehouse Management: Optimized warehouse operations, including receiving, putaway, picking, and shipping.
- Track and Trace Capabilities: Serialization and aggregation of products for end-to-end visibility.
Benefits of Implementing ERP in Pharmaceutical Supply Chain
Implementing an ERP system can bring numerous benefits to pharmaceutical companies:
- Improved Regulatory Compliance: ERP systems help companies meet regulatory requirements by automating compliance processes and providing audit trails.
- Enhanced Product Quality and Safety: By tracking raw materials, production processes, and finished goods, ERP systems help ensure product quality and safety.
- Reduced Costs: ERP systems can help reduce costs by optimizing inventory levels, streamlining production processes, and improving supply chain efficiency.
- Increased Efficiency: Automating tasks and providing real-time visibility into the supply chain can significantly increase efficiency.
- Better Decision-Making: ERP systems provide real-time data and analytics, enabling companies to make informed decisions.
- Improved Customer Service: By streamlining order management and providing accurate delivery information, ERP systems can improve customer service.
- Enhanced Collaboration: ERP systems facilitate collaboration among different departments and with external partners, such as suppliers and distributors.
- Reduced Risk of Counterfeiting: Track and trace functionalities inherent in many ERP systems provide better visibility and reduce the risk of counterfeit products entering the supply chain.
Challenges of ERP Implementation in Pharmaceuticals
While ERP systems offer significant benefits, implementing them in the pharmaceutical industry can be challenging. These challenges often stem from the complex regulatory environment, the need for data validation, and the resistance to change within the organization. From my experience, the biggest hurdle is often not the technology itself, but the change management aspect.

Common Implementation Challenges
- Data Migration: Migrating data from legacy systems to the ERP system can be complex and time-consuming. Data cleansing and validation are crucial to ensure data accuracy and integrity.
- System Integration: Integrating the ERP system with other systems, such as laboratory information management systems (LIMS) and manufacturing execution systems (MES), can be challenging.
- User Training: Training users on the new ERP system is essential for successful adoption. Adequate training resources and ongoing support are necessary.
- Change Management: Implementing an ERP system requires significant changes to business processes. Effective change management is crucial to overcome resistance and ensure user buy-in.
- Validation and Testing: Pharmaceutical companies must validate and test the ERP system to ensure it meets regulatory requirements. This process can be time-consuming and expensive.
- Scope Creep: The initial scope of the ERP implementation can expand over time, leading to delays and cost overruns. Careful planning and scope management are essential.
Overcoming Implementation Challenges
To overcome these challenges, pharmaceutical companies should:
- Develop a Comprehensive Implementation Plan: The plan should clearly define the scope, timeline, budget, and resources required for the implementation.
- Choose the Right ERP System: Select an ERP system that is specifically designed for the pharmaceutical industry and meets the company’s specific needs.
- Assemble a Strong Implementation Team: The team should include representatives from all key departments, as well as experienced ERP consultants.
- Prioritize Data Migration: Develop a data migration strategy that addresses data cleansing, validation, and transformation.
- Provide Adequate Training: Provide comprehensive training to all users on the new ERP system.
- Manage Change Effectively: Communicate the benefits of the ERP system to employees and involve them in the implementation process.
- Conduct Thorough Validation and Testing: Validate and test the ERP system to ensure it meets regulatory requirements.
- Establish Clear Scope Management Procedures: Define clear procedures for managing changes to the scope of the implementation.
Choosing the Right ERP Solution for Your Pharmaceutical Company
Selecting the right ERP system is a critical decision that can significantly impact the success of the implementation. Pharmaceutical companies should carefully evaluate their needs and requirements before choosing an ERP solution. Factors such as the size of the company, the complexity of its operations, and its regulatory requirements should be considered.
Key Considerations When Selecting an ERP System
- Industry-Specific Functionality: Ensure the ERP system offers functionality specific to the pharmaceutical industry, such as GMP compliance, batch management, and track-and-trace capabilities.
- Scalability: Choose an ERP system that can scale to meet the company’s future growth needs.
- Integration Capabilities: Ensure the ERP system can integrate with other systems, such as LIMS and MES.
- Vendor Reputation and Experience: Select a vendor with a strong reputation and experience in implementing ERP systems in the pharmaceutical industry.
- Total Cost of Ownership (TCO): Consider the total cost of ownership, including software licenses, implementation services, training, and ongoing maintenance.
- User-Friendliness: Choose an ERP system that is user-friendly and easy to learn.
- Security: Ensure the ERP system offers robust security features to protect sensitive data.
- Support and Maintenance: Evaluate the vendor’s support and maintenance offerings.
Popular ERP Systems for the Pharmaceutical Industry
Several ERP systems are popular in the pharmaceutical industry, including:
- SAP S/4HANA: A comprehensive ERP system that offers a wide range of functionality for the pharmaceutical industry.
- Oracle NetSuite: A cloud-based ERP system that is suitable for small and medium-sized pharmaceutical companies.
- Microsoft Dynamics 365: Another robust ERP solution that offers modularity and flexibility.
- Sage X3: An ERP system designed for mid-sized businesses, including those in the pharmaceutical sector.
Ultimately, the best ERP system for your pharmaceutical company will depend on your specific needs and requirements. It’s crucial to conduct thorough research and evaluation before making a decision.
Conclusion
Implementing an ERP system can be a transformative experience for pharmaceutical companies, enabling them to streamline their operations, improve regulatory compliance, and enhance their overall efficiency and profitability. However, successful implementation requires careful planning, effective change management, and a commitment to ongoing improvement. By understanding the challenges and taking the necessary steps to overcome them, pharmaceutical companies can leverage the power of ERP to gain a competitive edge in today’s demanding market.
Remember, ERP is not just a software solution; it’s a strategic investment that requires a holistic approach. It’s about transforming your business processes, empowering your employees, and creating a culture of continuous improvement. With the right ERP system and a dedicated team, your pharmaceutical company can achieve its full potential.
The journey to ERP implementation can be daunting, but the rewards are significant. By focusing on clear communication, user adoption, and continuous optimization, pharmaceutical companies can successfully navigate the complexities of ERP and unlock its full potential for driving growth and innovation.
Conclusion
In conclusion, the implementation of Enterprise Resource Planning (ERP) systems offers a transformative approach to pharmaceutical supply chain management. As we’ve explored, ERP streamlines operations, enhances visibility, and improves regulatory compliance across the entire supply chain – from raw material sourcing to finished product distribution. By integrating disparate functions, ERP systems facilitate better decision-making, reduce costs, and ultimately contribute to improved patient safety through enhanced product traceability and quality control. The benefits of a well-implemented ERP system are undeniable in today’s complex and highly regulated pharmaceutical landscape.
Moving forward, pharmaceutical companies should seriously consider investing in robust ERP solutions to optimize their supply chains and gain a competitive edge. The insights provided by ERP analytics, combined with proactive risk management strategies, will be crucial for navigating future challenges and ensuring the secure and efficient delivery of life-saving medications. If you are interested in learning more about how ERP can benefit your organization, we encourage you to contact our team for a personalized consultation and discover the potential for transformation within your pharmaceutical supply chain.
Frequently Asked Questions (FAQ) about Pharmaceutical Supply Chain Management through ERP
How does implementing an ERP system improve traceability in the pharmaceutical supply chain and why is traceability so important?
Implementing an ERP (Enterprise Resource Planning) system significantly enhances traceability in the pharmaceutical supply chain by providing a centralized and integrated platform for managing data across all stages, from raw material sourcing to finished product distribution. This visibility allows pharmaceutical companies to track products at every point, knowing the origin of materials, the manufacturing processes involved, and the distribution routes. This is achieved through features like batch tracking, serial number management, and real-time data capture. Managing these diverse processes efficiently often necessitates a comprehensive solution, ERP being one such system designed for integrated business management
.
Traceability is critical in the pharmaceutical industry because it enables rapid identification and recall of potentially harmful or substandard products. This protects patient safety and brand reputation. Furthermore, it supports compliance with stringent regulatory requirements like the Drug Supply Chain Security Act (DSCSA) in the US, which mandates end-to-end traceability to combat counterfeit drugs. Improved traceability also leads to better inventory management, reduced waste, and enhanced operational efficiency.
What are the key challenges pharmaceutical companies face when integrating an ERP system into their existing supply chain, and how can these challenges be overcome?
Integrating an ERP system into a pharmaceutical supply chain presents several challenges. One major hurdle is the complexity of the pharmaceutical industry, with its stringent regulatory requirements (e.g., FDA regulations like 21 CFR Part 11), diverse product lines, and intricate manufacturing processes. Data migration from legacy systems can also be complex and prone to errors, leading to inconsistencies and inaccuracies. Another challenge is resistance to change from employees accustomed to existing processes.
To overcome these challenges, companies should prioritize thorough planning and preparation. This includes conducting a comprehensive needs assessment to identify specific requirements, selecting an ERP system specifically designed for the pharmaceutical industry, and developing a detailed implementation plan. Investing in robust data cleansing and migration strategies is crucial. Furthermore, providing comprehensive training to employees and fostering a culture of collaboration and communication can minimize resistance and ensure successful adoption. A phased implementation approach can also mitigate risks and allow for adjustments along the way. Proper validation of the ERP system is also critical to meet regulatory needs.
What specific ERP modules are most critical for managing a pharmaceutical supply chain effectively, and what benefits do they offer?
Several ERP modules are essential for effective pharmaceutical supply chain management. Inventory management modules are crucial for tracking raw materials, work-in-progress, and finished goods, optimizing stock levels, and minimizing waste. Manufacturing execution system (MES) integration provides real-time visibility into production processes, ensuring quality control and compliance with Good Manufacturing Practices (GMP). Quality management modules help manage quality control processes, track deviations, and ensure product safety and efficacy.
Furthermore, supply chain planning modules enable demand forecasting, capacity planning, and production scheduling, optimizing resource allocation and minimizing lead times. Warehouse management modules streamline warehouse operations, improve inventory accuracy, and reduce picking and shipping errors. Finally, Regulatory compliance modules, designed to maintain adherence to industry regulations and standards, are essential. These modules offer benefits such as improved efficiency, reduced costs, enhanced quality control, and increased transparency across the supply chain, ultimately leading to better patient outcomes and regulatory compliance.